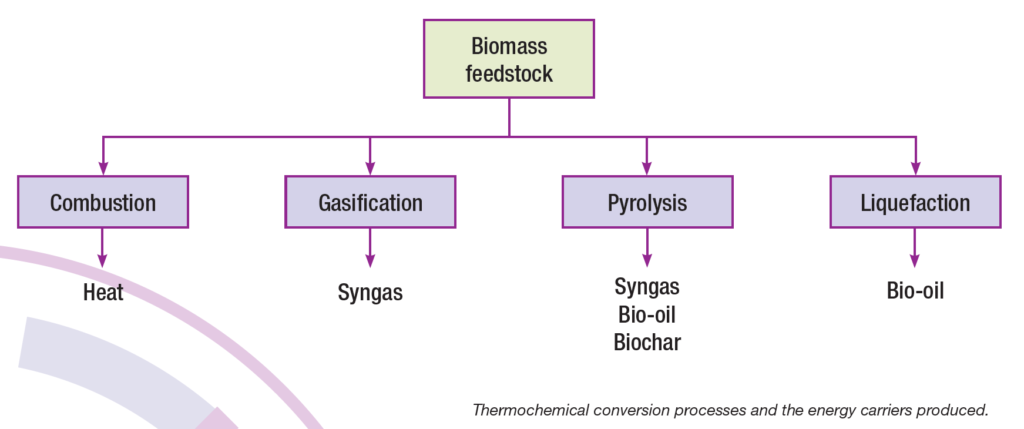
Lignocellulosic biomass can be converted into fuels and chemicals using thermochemical or biochemical process pathways. Thermochemical technologies apply heat and chemical processes in order to produce bioenergy from biomass. There are four main thermochemical conversion processes: direct combustion, gasification, pyrolysis and liquefaction. Direct combustion produces heat while the three latter can produce various types of energy carriers that can be converted into fuels.
Direct combustion
Direct combustion is the burning of biomass in open air, or, in the presence of excess air, converting the chemical energy stored in biomass into heat, mechanical power or electricity. Direct combustion is carried out using stoves, furnaces, steam turbines, or boilers at a temperature range starting at 800°C. All types of biomass can be burned, but in practice, direct combustion is only performed for biomass that has low moisture content (less than 50%). Biomass containing higher levels of moisture needs to be dried prior to combustion, or it may be better suited to biochemical conversion.
Gasification
Gasification is the partial oxidation of biomass at high temperatures (over 700°C) in the presence of a gasification agent, which can be steam, oxygen, air or a combination of these. The resulting gas mixture is called syngas or producer gas, and can be used in various processes to produce liquid fuels such as methanol, ethanol and Fischer-Tropsch diesel, and gaseous fuels, such as hydrogen and methane.
Syngas is comprised mainly of hydrogen and carbon monoxide, but could also contain methane, carbon dioxide, light hydrocarbons (e.g. ethane and propane) and heavy hydrocarbons (e.g. tars). Undesirable gases, such as hydrogen sulfide may also be present. The composition of the syngas depends on the type of biomass, the gasifier, the gasification agent, and on the temperature used in the process. Generally, when the biomass has high content of carbon and oxygen, the syngas produced via gasification is rich in carbon monoxide and carbon dioxide.
The most common biomass feedstocks used in the gasification process to produce biofuels are different kinds of wood, forestry wastes and agricultural residues. The heat for the high temperature gasification process can be supplied either directly by oxidation of part of the biomass in the gasifier, or indirectly by transferring energy to the gasifier externally.
Pyrolysis
Pyrolysis is the thermal decomposition of biomass to liquid, solid and gaseous fractions at high temperatures in the absence of oxygen in order to avoid significant levels of combustion. The liquid fraction is called bio-oil or bio-crude; a dark brown, viscous liquid with a high density, composed by a mixture of oxygen-containing organic compounds. Due to its high oxygen content, bio-oil is not suitable for direct use as a drop-in transportation fuel. However, it can be easily transported and stored, and after upgrading it has the potential to substitute crude oil, which makes it the most interesting product of pyrolysis. The solid fraction obtained from pyrolysis is called biochar, i.e. charcoal made from biomass, and the gasous fraction is syngas. The relative proportions of these fractions depend on the type of reactor employed and the feedstock used. It is controlled by varying the temperature, the heating rate and the residence time of the material in the reactor.
Depending on the heating rate employed, there are three main types of pyrolysis processes: slow, fast and flash pyrolysis. Slow pyrolysis has been used for thousands of years for the production of solid fuel. It is a decomposition process at relatively low temperatures (up to 500°C) and low heating rates (below 10°C/min), which takes several hours to complete, and results in solid biochar as the main product.
Fast pyrolysis is currently the most widely used process. It occurs at controlled temperature of around 500°C employing relatively high heating rates and only takes a few seconds to complete. The key product from fast pyrolysis is bio-oil (60-75%). In addition, biochar (15-25%) and syngas (10-20%) are also produced.
When heating rates and reaction temperatures are even higher, and the reaction time is shorter than that of fast pyrolysis, the process can be described as flash pyrolysis. Flash pyrolysis can result in a high yield of bio-oil and high conversion efficiencies (up to 70-75%).
Liquefaction
Hydrothermal liquefaction is the conversion of biomass to bio-oil in the presence of water, with or without a catalyst. During hydrothermal liquefaction, large compounds in the biomass are broken down into unstable shorter molecules that in turn reattach to each other and form bio-oil. In contrast to pyrolysis and gasification, the liquefaction process does not require the use of dry biomass, which reduces the cost of drying. The resulting bio-oil has lower oxygen content than the bio-oil obtained from pyrolysis, and therefore, it requires less upgrading prior to utilization as a transportation fuel.